شرح Analytical chemistry 2 first year first level practical activity | [معتمد]
دورة الفرقة الأولى Analytical chemistry 2
شارك الآن استفساراتك مع اعضاء دورة الفرقة الأولى Analytical chemistry 2 اضغط هنا
سجل الآن
قائمة الدروس | 44 درس
التعليقات
دورات ذات صلة
دورة معتمدة اون لاين مجانية Analytical chemistry studies and uses instruments and methods used to separate, identify, and quantify matter.[1] In practice, separation, identification or quantification may constitute the entire analysis or be combined with another method. Separation isolates analytes. Qualitative analysis identifies analytes, while quantitative analysis determines the numerical amount or concentration. Analytical chemistry is the science of obtaining, processing, and communicating information about the composition and structure of matter. In other words, it is the art and science of determining what matter is and how much of it exists. ... It is one of the most popular fields of work for ACS chemists.
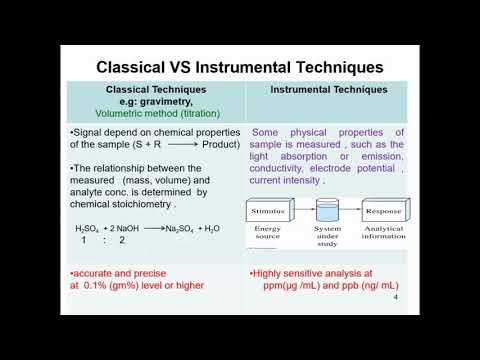
Analytical chemistry consists of classical, wet chemical methods and modern, instrumental methods.[2] Classical qualitative methods use separations such as precipitation, extraction, and distillation. Identification may be based on differences in color, odor, melting point, boiling point, solubility, radioactivity or reactivity. Classical quantitative analysis uses mass or volume changes to quantify amount. Instrumental methods may be used to separate samples using chromatography, electrophoresis or field flow fractionation. Then qualitative and quantitative analysis can be performed, often with the same instrument and may use light interaction, heat interaction, electric fields or magnetic fields. Often the same instrument can separate, identify and quantify an analyte.
Analytical chemistry is also focused on improvements in experimental design, chemometrics, and the creation of new measurement tools. Analytical chemistry has broad applications to medicine, science, and engineering. قناة كلية الصيدلة جامعة طنطا the first group